Registry
Transnational Fracture Registry Platform
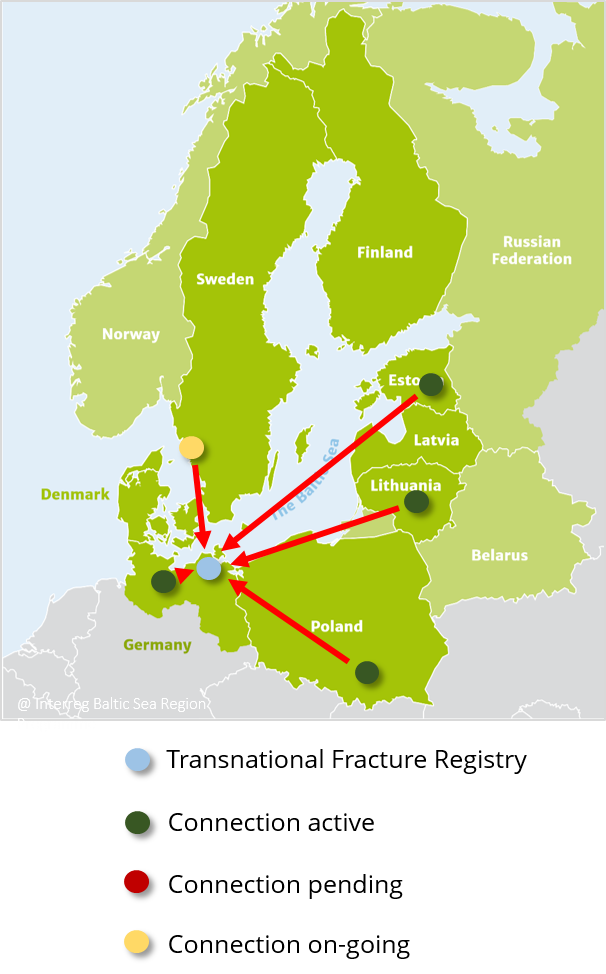
The BFCC’s Transnational Fracture Registry Platform (TFRP) is the core element necessary for the acquisition, processing, integration, storage and analysis of homogenous fracture registry data. In December 2017 it was launched by the University Medicine Greifswald at production level.
Aiming to compare the process and outcome quality across institutions and countries, the BFCC’s partnering hospitals from the Baltic Sea Region provide the registry with necessary patient data.
This transnational research and innovation infrastructure will foster the evidence-based identification of clinical best practice and innovation needs.
- Click here to log in to the registry (the registry is only accessible for already connected hospitals).
Feedback desired: Core Event Set for Fracture Complication Registry
Within the fracture registry there is also the documentation of post-surgery complications within fracture treatment. The project partners have developed a "Core Event Set" for this purpose. In the pilot phase, a Delphi process is used to determine which elements are to be included. Stakeholders are invited to evaluate the set. The suggestions will be considered and help to develop a classification system.
How to get access to the BFCC registry?
Common Minimal Data Set
The uniform specification of a common minimal data set (CMD) plays a vital role when it comes to using and operating with given data on a transnational level. The CMD is the mandatory data set for all participating hospitals being collected and entered to the registry. The BFCC consortium has identified several items for the CMD aiming to gather the basic needed information for each entered patient. Furthermore, every hospital is free to enter additional data about a patient useful, e. g. for the specific purpose within a certain type of fracture.
Regarding the different interests of involved groups in the project and external people, it is planned to frequently review the data set by external people in order to keep the registry sustainable. We developed a questionnaire with the aim to get feedback from different point of views to better adapt and change current versions.
- Click here to get an impression of the Minimal Data Set.
- Click here and be part of our quality management by filling out the CMD questionnaire.
MDR Feasibility Study and Implant Related Data Set
In order to address current changes in the regulations, the partners integrated a feasibility study in the running project: Enlargement of the pilot study ‘complication’ to embed a possibility to access routine registry data with the purpose to include specific medical device data.
Automated Registry Reporting
To enable continuous surveillance of data from the transnational fracture registry platform and to compare the process’ quality and health outcome related to relevant clinical issues, the BFCC partners have developed an automatic reporting system with a specific algorithm.